Stanford and SLAC scientists have redesigned current conductors – thin metal foils that distribute power to and from electrodes – to make lithium-ion batteries lighter, safer, and more efficient. They replaced the full copper conductor in the middle with a layer of light polymer coated with ultra-thin copper (top right) and with a fire retardant embedded in the polymer layer to extinguish flames (bottom right). Photo credit: Yusheng Ye / Stanford University
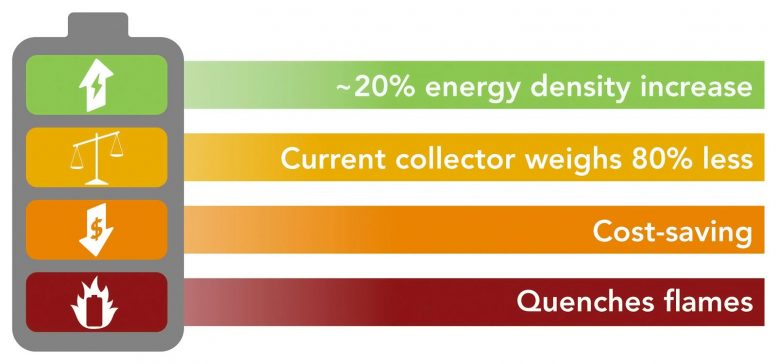
Adding polymers and fire protection to a battery’s current collectors makes it lighter, safer, and about 20% more efficient.
In a completely new approach to making lithium-ion batteries lighter, safer, and more efficient, scientists from Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory have reworked one of the heaviest battery components – copper or aluminum foils, known as current collectors – to weigh them 80% less and immediately extinguish any fires that start up.
If acquired, these technologies could achieve two main goals for battery research: extending the range of electric vehicles and reducing the risk of laptops, cell phones, and other devices going up in flames. This is especially important when charging batteries super-fast, which creates more types of battery damage that can lead to fire.
The research team described their work in Natural energy on October 15, 2020.
“The current collector has always been considered deadweight and has not been used successfully to increase battery performance,” said Yi Cui, professor at SLAC and Stanford and researcher at the Stanford Institute for Materials and Energy Sciences (SIMES), who conducted the research directed.
“In our study, the energy density of lithium-ion batteries – how much energy they can store in a given weight – was increased by 16 to 26% by making the collector 80% lighter. That’s a big jump compared to the 3% average increase over the past few years. ”
Compared to current collectors of today’s lithium-ion batteries, a new version developed by scientists from Stanford and SLAC makes batteries lighter, more energy efficient and safer. It could also cut costs by replacing copper with cheaper polymer and lowering the cost of shipping batteries for recycling. Photo credit: Greg Stewart / SLAC National Accelerator Laboratory
Desperate for weight loss
Regardless of whether it is a cylinder or a bag, lithium-ion batteries have two current collectors, one for each electrode. They distribute the current flowing in or out of the electrode and make up 15% to 50% of the weight of some high-performance or ultra-thin batteries. Saving the weight of a battery is desirable in itself to enable lighter equipment and to reduce the weight that electric vehicles have to lug around. By storing more energy per given weight, both devices and electric vehicles can wait longer between charges.
Reducing battery weight and flammability could also have a big impact on recycling as it makes it cheaper to ship recycled batteries, Cui said.
Researchers in the battery industry have tried to reduce the weight of current collectors by making them thinner or more porous. However, these attempts had undesirable side effects such as: For example, battery sensitivity is more fragile or chemically unstable, or the need for more electrolyte, which increases costs, said Yusheng Ye, a postdoctoral fellow at Cui’s laboratory who conducted the experiments with visiting scientist Lien-Yang Chou.
Regarding safety, he said, “People have also tried to add fire retardant to the battery electrolyte, the flammable part, but you can only add so much before it becomes viscous and no longer conducts ions well.”

In a study at Stanford and SLAC, lithium-ion pouch batteries made with today’s commercial current collectors (top row) ignited fire when exposed to an open flame and burned violently until all of the electrolyte was burned away. Batteries with the new flame-retardant collectors (bottom row) produced weak flames that went out within a few seconds and didn’t flare up again even when scientists tried to rekindle them. Photo credit: Yusheng Ye / Stanford University
Design a polymer film sandwich
After brainstorming the problem, Cui, Ye, and PhD student Yayuan Liu designed experiments to make and test current collectors based on a lightweight polymer called polyimide, which can withstand fire and the high temperatures caused by rapidly charging the battery. A fire retardant – triphenyl phosphate, or TPP – was embedded in the polymer, which was then coated with an ultra-thin layer of copper on both surfaces. Not only would the copper do its usual job of distributing electricity, but it would also protect the polymer and its fire retardant.
These changes reduced the weight of the current collector by 80% compared to today’s versions, Ye said, resulting in an increase in energy density of 16-26% for various types of batteries and conducting electricity just as well as regular collectors without degradation.
When pouch batteries made with today’s commercial power collectors were exposed to an open flame from a lighter, they would ignite and burn violently until all of the electrolyte was burned away, Ye said. However, with batteries with the new flame retardant collectors, the fire never really started, producing very weak flames that went out within a few seconds and didn’t flare up even when scientists tried to rekindle it.
One of the great advantages of this approach, says Cui, is that the new collector should be easy to manufacture and also cheaper because it replaces some of the copper with an inexpensive polymer. Scaling up for commercial production, he said, “should be very doable.” The researchers have applied for a patent through Stanford and Cui said they would contact the battery manufacturers to explore the possibilities.
Reference: “Ultra-light and fire-extinguishing current collectors for high-energy and high-security lithium-ion batteries” by Yusheng Ye, Lien-Yang Chou, Yayuan Liu, Hansen Wang, Hiang Kwee Lee, Wenxiao Huang, Jiayu Wan and Kai Liu, Guangmin Zhou, Yufei Yang , Ankun Yang, Xin Xiao, Xin Gao, David Thomas Boyle, Hao Chen, Wenbo Zhang, Sang Cheol Kim and Yi Cui, October 15, 2020, Natural energy.
DOI: 10.1038 / s41560-020-00702-8
This work was supported by the DOE Energy Efficiency and Renewable Energy Office, Vehicle Technologies Office, as part of the eXtreme Fast Charge Cell Evaluation of Lithium-Ion Batteries (XCEL) program.



