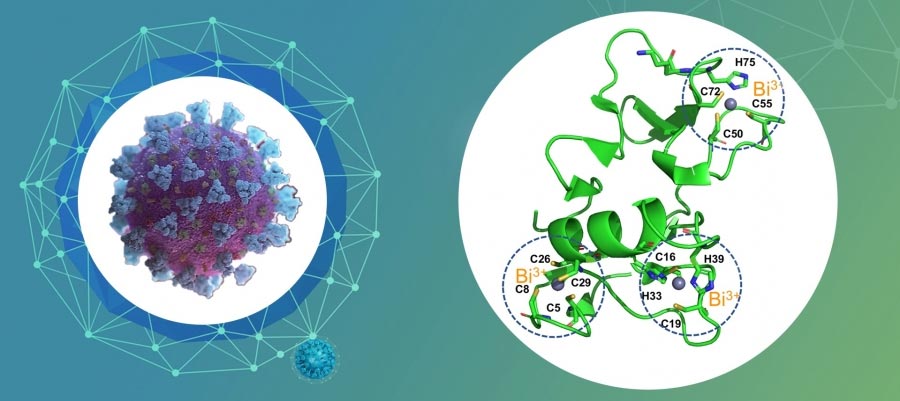
Proposed structure of the bi-bonded zinc-binding domain of the SARS-CoV-2 helicase. By switching off the crucial zinc (II) ions in the zinc binding domain of the SARS-CoV-2 helicase, RBC demonstrated its ability to effectively suppress the replication of SARS-CoV-2. Credit: The University of Hong Kong
HKU scientists and microbiologists are jointly discovering a novel antiviral strategy for the treatment of COVID-19 using existing metal drugs.
A research team led by Professor Hongzhe SUN, Norman & Cecilia Yip, Professor of Bioinorganic Chemistry, Department of Chemistry, Faculty of Science, and Professor Kwok Yung YUEN, Henry Fok, Professor of Infectious Diseases, Department of Microbiology, Li Ka Shing, Faculty for Medicine The University of Hong Kong (HKU) has discovered a novel antiviral strategy to treat COVID-19.
They discovered that a class of metal drugs currently used to treat other infectious diseases show effective suppression SARS-CoV-2 Replication and alleviation of virally associated symptoms in an animal model.
The results offer a new and readily available treatment option with high clinical potential for infection with SARS-CoV-2. This groundbreaking work was published online in a high profile scientific journal Natural microbiology. A corresponding patent has been applied for in the USA.

From right: Dr. Jasper FW CHAN and Dr. Shuofeng YUAN from the Department of Microbiology, Professor Hongzhe SUN and Dr. Runming WANG from the Department of Chemistry. Credit: The University of Hong Kong
background
SARS-CoV-2 is a emerging coronavirus that has caused over 30 million laboratory-confirmed cases and more than 1 million deaths from COVID-19 worldwide since December 2019. With the development of an effective vaccine ongoing, another approach to prevention and treatment for the disease is to identify anti-COVID-19 agents from existing virus-specific antiviral drugs in order to reuse their use to fight the new virus . Remdesivir, a broad spectrum antiviral drug, has been reported to be effective against SARS-CoV-2. However, the global shortage of the drug, its relatively high price, and the lack of significant clinical benefits in severe cases are factors that have limited its wider uses. Clinical studies are still ongoing with a number of antiviral agents that are not yet therapeutically effective. Therefore, greater efforts are needed to expand the evaluation to a wider range of clinically approved drugs, which hopefully could pave the way for alternative treatment strategies for the disease through some readily available channels.
Investigation method and results
In general, metal compounds are used as antimicrobial agents; Their antiviral activities have rarely been studied. After screening a number of metallodrugs and related compounds, the research team identified ranitidine bismuth citrate (RBC), a commonly used anti-ulcer drug that contains the metal bismuth to treat Helicobacter pylori-associated infections, as an effective anti-SARS-CoV-2 Agent, both in vitro and in vivo.
RBC targets the vital non-structural protein 13 (Nsp13), a viral helicase essential for SARS-CoV-2 replication by displacing the critical zinc (II) ions in the zinc bond with bismuth for activity effective to suppress the helicase.
RBC has been shown to greatly reduce viral load in SARS-CoV-2 infected cells by 1000-fold. In a Syrian golden hamster model in particular, RBC suppresses SARS-CoV-2 replications to reduce viral loads in both the upper and lower airways by ~ 100-fold and to alleviate virus-associated pneumonia. RBC significantly reduces the levels of prognostic markers and other important pro-inflammatory cytokines and chemokines in severe COVID-19 cases of infected hamsters compared to the remdesivir-treated and control groups.
RBC shows low cytotoxicity with a high selectivity index at 975 (the larger the number, the safer the drug) compared to remdesivir which has a low selectivity index at 129. The finding shows a wide window between the drug’s cytotoxicity and its antiviral activity. This allows great flexibility in adjusting the dosages for the treatment.
The team examined the mechanisms of RBC on SARS-CoV-2, and for the first time revealed the vital Nsp13 helicase as a drugable target of RBC. It irreversibly ejects the crucial zinc (II) ions in the zinc binding domain in order to convert them into bismuth-bound ones via a certain metal displacement path. RBC and its Bi (III) compounds defunctionalized the Nsp13 helicase and inhibited both ATPase (IC 50 = 0.69 µM) and effectively DNA– Unwinding activities (IC 50 = 0.70 µM) of this enzyme.
The research highlights viral helicases as a drugable target and the high clinical potential of bismuth (III) drugs and other metallodrugs for the treatment of SARS-CoV-2 infections. After this important breakthrough, hopefully more antivirals could be identified from readily available clinically approved drugs for potential treatment of COVID-19 infections. They can be in the form of combination schemes (cocktails) with drugs that have anti-SARS-CoV-2 activities, including RBC, dexamethasone, and interferon-β1b.
Reference: “Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and alleviates virus-associated pneumonia in Syrian hamsters” by Shuofeng Yuan, Runming Wang, Jasper Fuk-Woo Chan, Anna Jinxia Zhang, Tianfan Cheng, Kenn Ka-Heng Chik, Zi -Wei Ye, Suyu Wang, Andrew Chak-Yiu Lee, Lijian Jin, Hongyan Li, Dong-Yan Jin, Kwok-Yung Yuen and Hongzhe Sun, October 7th, 2020, Natural microbiology.
DOI: 10.1038 / s41564-020-00802-x
Key members of the research team:
Institute of Chemistry, Faculty of Natural Sciences
- Professor Hongzhe SUN, Norman & Cecilia Yip Professor of Bioinorganic Chemistry, Chair of Chemistry
- Dr. Runming WANG, Postdoctoral Researcher, Department of Chemistry
Department of Microbiology, Li Ka Shing Medical School
- Professor Kwok-Yung YUENHenry Fok Professor of Infectious Diseases; also Co-Director of the State Key Laboratory of Emerging Infectious Diseases and Academic of the Chinese Academy of Engineering
- Dr. Shuofeng YUAN, Research Associate Professor
- Dr. Jasper FW CHAN, Clinical Assistant Professor
Staunch supporters
The work was supported by the Research Grants Council (RGC) in Hong Kong, the National Key R&D Programs in China, the Seed Fund of the University of Hong Kong (HKU) for basic research and donations from the Lo Ying Shek Chi Wai Foundation, Richard Yu and Carol Yu, Hong Kong Shaw Foundation, Michael Seak-Kan Tong, May Tam Mak Mei Yin, Hong Kong Sanatorium and Hospital, Hui Ming, Hui Hoy, and Chow Sin Lan Charity Fund Limited, Chan Yin Chuen Memorial Charitable Foundation , Marina Man-Wai Lee, the South China Microbiology Research Fund of the Hong Kong Hainan Commercial Association, the Jessie & George Ho charitable foundation, the Perfect Shape Medical Limited, the Kai Chong Tong, the Foo Oi Foundation Limited, the Tse Kam Ming Laurence and the Norman & Cecilia Yip Foundation.