By
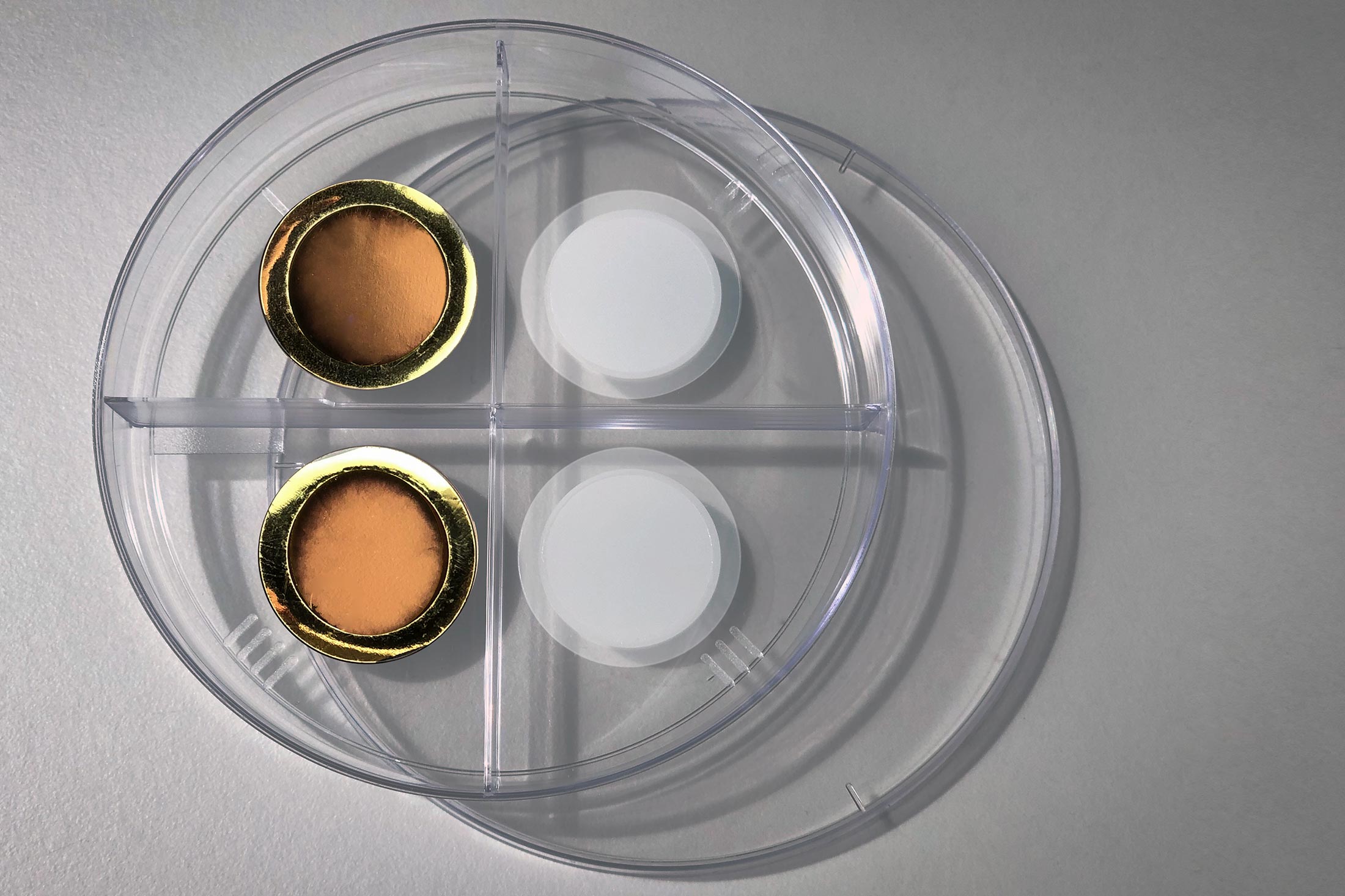
On the right is a porous anodized aluminum oxide membrane. The left side shows the same membrane after it has been coated with a thin layer of gold, which makes the membrane conductive for electrochemical gas control. Photo credit: Felice Frankel
The electrically switchable system can continuously separate gases without the need for moving parts or wasted space.
A new system developed by chemical engineers at WITH could offer a way to continuously remove carbon dioxide from an exhaust gas stream or even from the air. The key component is an electrochemically assisted membrane, the gas permeability of which can be switched on and off at will, with no moving parts and relatively little energy.
The membranes themselves, made of anodized aluminum oxide, have a honeycomb-like structure of hexagonal openings through which gas molecules can flow in and out when open. However, the passage of gas can be blocked if a thin layer of metal is electrodeposited to cover the pores of the membrane. The work is described in an article published in the journal by Professor T. Alan Hatton, Postdoc Yayuan Liu, and four others Advances in science on October 16, 2020.
This new “gas gating” mechanism could be used to continuously remove carbon dioxide from a range of industrial waste gas streams and from the ambient air, the team said. They built a proof of concept device to show this process in action.
The device uses a redox-active carbon-absorbing material, which is arranged between two switchable gas gate membranes. The sorbent and gate membranes are in intimate contact with one another and are immersed in an organic electrolyte to provide a medium in which zinc ions can shuttle back and forth. These two gate membranes can be opened or closed electrically by switching the polarity of a voltage between them, causing zinc ions to shuttle from one side to the other. The ions simultaneously block one side by forming a metal film over it, while opening the other by peeling away its film.
If the sorbent layer is open to the side where the exhaust gases pass, the material will easily pick up carbon dioxide until it reaches its capacity. The voltage can then be switched to block the supply side and open the other side where a concentrated stream of nearly pure carbon dioxide is released.
By building a system with alternating membrane sections operating in opposite phases, the system would allow for continuous operation in an environment such as an industrial scrubber. At any point in time, half of the sections would absorb the gas while the other half would release it.
“That means there is a feed stream going into the system at one end and the product stream flowing out of the other in what appears to be a continuous operation,” says Hatton. “This approach avoids many of the process problems” that would be encountered in a conventional multi-column system in which adsorbent beds must be alternately shut down, purged, and then regenerated before re-exposure to feed gas to begin the next adsorption cycle. In the new system, the flushing steps are not required and all steps are neatly done inside the device.
The researchers’ key innovation was electroplating to open and close the pores of a material. On the way, the team tried various other approaches to reversibly close pores in a membrane material, such as the use of tiny magnetic balls that could be positioned so that funnel-shaped openings were blocked. However, these other methods were not found to be efficient enough. Metal thin films can be particularly effective as gas barriers, and the ultra-thin layer used in the new system requires a minimal amount of the zinc material, which is abundant and inexpensive.
“It makes a very uniform coating layer with a minimal amount of materials,” says Liu. A major advantage of the electroplating process is that after changing the state in the open or closed position, no energy input is required to maintain this state. Energy is only required to switch back.
Such a system could possibly make an important contribution to limiting emissions of greenhouse gases into the atmosphere and even to the direct capture of already emitted carbon dioxide from the air.
While the team initially focused on the challenge of separating carbon dioxide from a gas stream, the system could actually be adapted to a variety of chemical separation and purification processes, says Hatton.
“We’re pretty excited about the gate mechanism. I think we can use it in a wide variety of applications and in different configurations, ”he says. “Maybe in microfluidic devices, or maybe we could use it to control the gas composition for a chemical reaction. There are many different options. ”
Reference: “Electrochemically Mediated Gate Membrane with Dynamically Controllable Gas Transport” by Yayuan Liu, Chun-Man Chow, Katherine R. Phillips, Miao Wang, Sahag Voskian and T. Alan Hatton, October 16, 2020, Advances in science.
DOI: 10.1126 / sciadv.abc1741
The research team consisted of PhD student Chun-Man Chow, postdoc Katherine Phillips, and recent graduates Miao Wang PhD ’20 and Sahag Voskian PhD ’19. This work was supported by ExxonMobil through the MIT Energy Initiative.