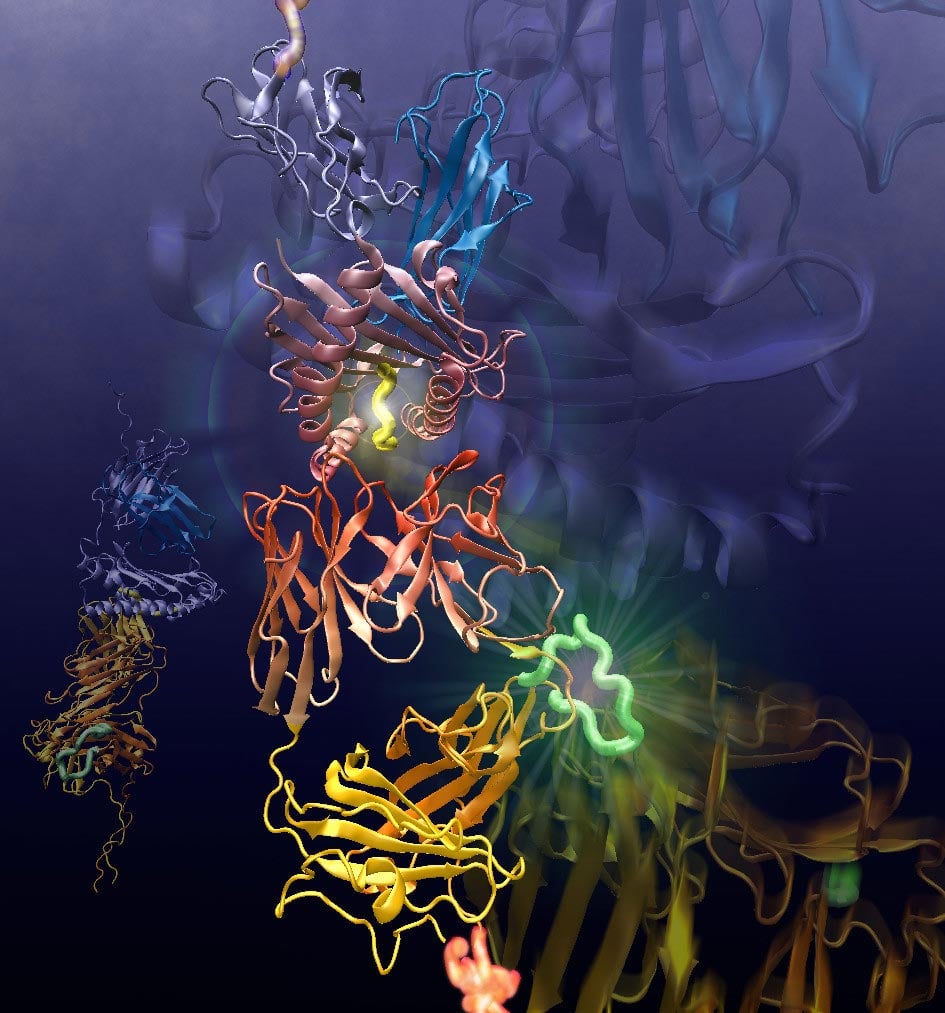
In this picture there is a T cell receptor on the lower side, and the main histocompatibility complex on the upper side shows the antigen colored yellow in the middle. The simulation made it possible for Dr. Wonmuk Hwang to analyze the reaction of the complex under mechanical stress. Photo credit: Dr. Wonmuk Hwang, Texas A&M University College of Engineering
Research, led by Wonmuk Hwang, Associate Professor at Texas A&M University, has led to a better understanding of how components of the body’s immune system find invading or damaged cells, which could lead to novel approaches to treating viruses and cancer.
Hwang, Associate Professor in the Department of Biomedical Engineering at Texas A&M University, wrote about this in a recent article in the journal Procedure of the National Academy of Sciences.
When viruses enter the body, the immune system goes into gear to seek out and destroy the invader. T cells are a component of the immune system and look for viruses that hide in host cells and serve as the ultimate line of defense against antigens or foreign bodies. T cells examine the surface of other cells and examine materials taken from inside the cell and presented by the MHC (Major Histocompatibility Complex) molecules on the surface of the cells.
“The problem is that hundreds of thousands of MHC molecules have peptides and few, if any, come from invading cells,” Hwang said. “The rest of them are normal products of cell metabolism, which means the T-cell needs to see that needle in a haystack.”
Researchers have discovered that T cells mechanically increase their recognition performance: When T cells examine the surface of other cells, a natural contact force is created. When the cell is infected with an antigen, the force applied creates a “captive bond” between the T cell receptors (TCRs) and the MHC molecules, thereby increasing contact. This binding does not occur between TCRs and MCH molecules that do not carry specific antigens.
However, it is almost impossible to see this interaction experimentally in atomic detail. Therefore, Hwang developed a computer simulation with which the interaction between TCRs and MHC molecules can be realistically demonstrated and analyzed when a force is applied.
“Only the simulation can see and analyze molecular movements under load. A laboratory experiment doesn’t have the answer, ”said Hwang. “Experimentally determined atomic structures of proteins are static snapshots, but when the molecule moves, you basically cannot see the movement.”
What Hwang discovered was how the movement between the parts of the TCR controls their interaction with the MHC molecules. When force is applied, movement is only suppressed if the MHC molecule has the appropriate antigen, thereby stabilizing the entire complex. Other cases refuse to lock onto the TCR, and the constant movement between the two eventually causes them to separate. It is like a lock-and-key system, in which the lock and key constantly change their shape and the molecules can only interlock if they match perfectly and are adequately strong.
Hwang said knowing which parts of the molecule respond to force can help tailor T cells for specific uses. In addition to fighting infections, TCRs are also the rising stars of cancer therapy.
“If you can train the T cell to see these cancerous antigens, it will be really specific therapy,” Hwang said. “Chemotherapy kills all cells. But T cells can train you to detect cancer cells with extremes accuracy. ”
Hwang said the next step is to study what is general and what relates to specific T cell receptor systems.
“To see how this principle applies to different T-cell receptors, I’ll expand on this initial finding,” said Hwang. “This is the very first piece of work that has put the mechanism of action of T-cell receptors into effect.”
Reference: “The αβTCR Mechanosensor Uses Dynamic Ectodomain Allostery to Optimize Its Ligand Recognition Site” by Wonmuk Hwang, Robert J. Mallis, Matthew J. Lang and Ellis L. Reinherz, August 13, 2020 Procedure of the National Academy of Sciences.
DOI: 10.1073 / pnas.2005899117
Hwang worked with researchers from Vanderbilt University and Harvard Medical School, and the research was supported by grants from the National Institutes of Health.