By
Mice lacking CNOT3 in pancreatic beta cells have fewer insulin-producing cells, which leads to diabetes. Image credit: OIST
A protein found throughout the body plays a key role in regulating glucose levels, according to a new study conducted in the Cell Signal Unit at the Graduate University of the Okinawa Institute of Science and Technology (OIST) and the Riken Center of Integrative Medical Sciences has been. This protein, called CNOT3, was found to silence a number of genes that would otherwise cause insulin-producing cells to malfunction, which is linked to the development of diabetes.
Diabetes is a common condition that causes very high blood sugar levels. If left untreated, it can cause serious health problems such as kidney failure, heart disease, and loss of vision. This disorder occurs when there is not enough insulin in the body or when the insulin-induced responses are weakened. Insulin normally lets glucose into cells for energy use, and without it, glucose builds up in the blood instead. Insulin deficiency is often caused by the pancreatic beta cells that normally synthesize and secrete insulin no longer working properly.
“We know that defects in beta cells can lead to high levels of glucose in the blood and ultimately to diabetes.” said Dr. Dina Mostafa, former doctoral student in the department and first author of the in Communication biology. “Our results suggest that CNOT3 is involved and plays a key role in the maintenance of normal beta cell function.”
Switching off CNOT3 leads to diabetes in mice:
CNOT3 is an all-rounder. It is expressed by many organs throughout the body, and it regulates different genes in different tissues. But its activity has a common basis – it helps keep cells alive, healthy, and functioning properly. This happens through various mechanisms, for example through the production of the right proteins or the suppression of certain genes.
Here, the researchers examined its function in islet cells from pancreatic tissue in mice. These islands are known to be difficult to work with. They only make up one to two percent of the pancreas, but that’s where the beta cells are.

The researchers from the Cell Signal Unit at OIST. From left Dr. Akiko Yanagiya, Dr. Dina Mostafa and Professor Tadashi Yamamoto. Image credit: OIST
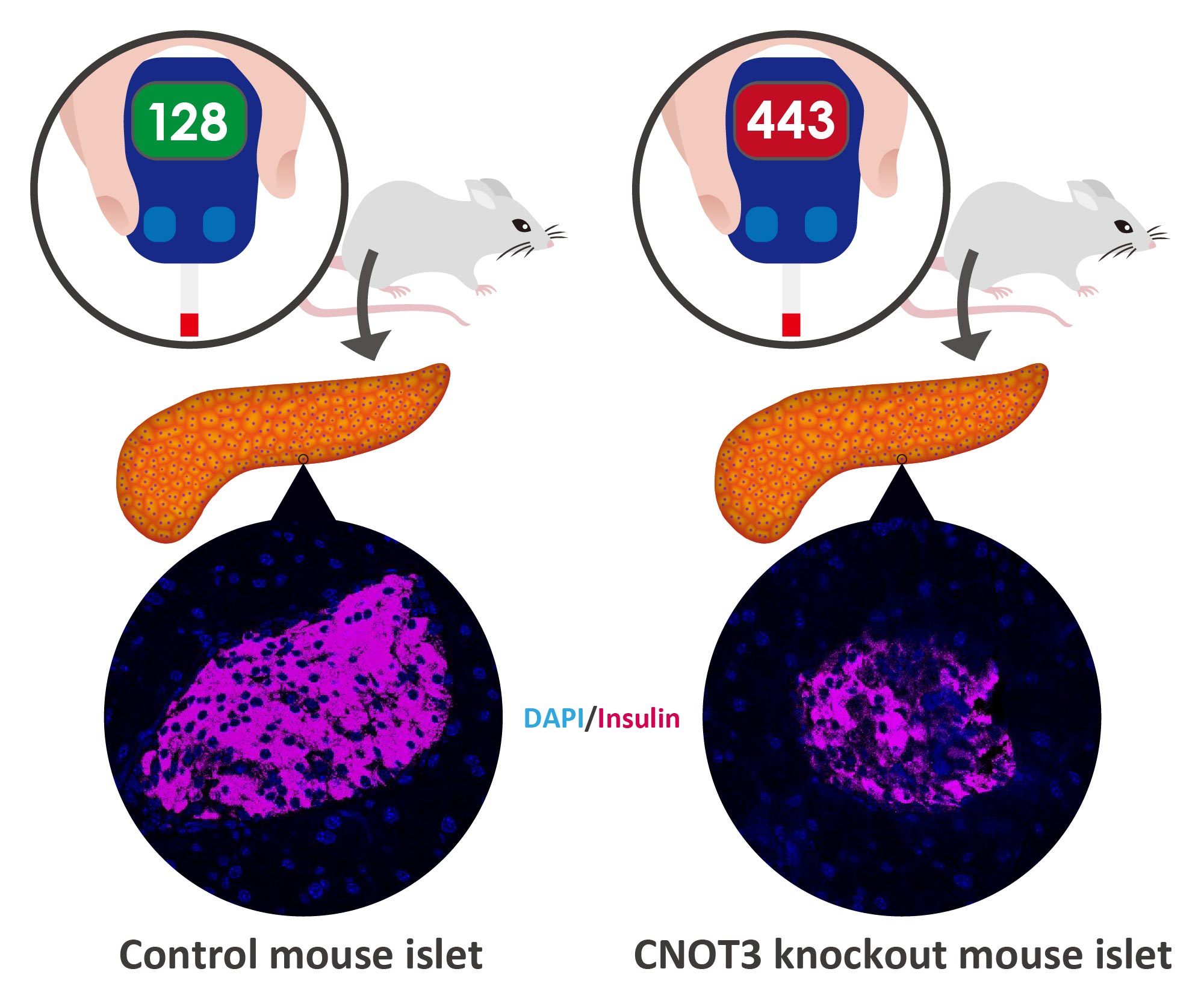
The researchers first investigated whether CNOT3 expression in diabetic mice was different than in non-diabetic mice. Looking at these islets, they found that the CNOT3 decreased significantly in the diabetic islets in contrast to the non-diabetic islets.
To further investigate the function of the protein, the researchers blocked its production in the beta cells of otherwise normal mice. The animals’ metabolism was normal for four weeks, but by week eight they had developed intolerance to glucose and by week twelve they had full blown diabetes.
Without CNOT3, the researchers found that some genes that are normally turned off in beta cells turn on and start producing proteins. Under normal circumstances, these genes are silenced as they cause all sorts of problems for the beta cells when turned on, e.g. B. the prevention of insulin secretion in response to glucose.
“We still don’t know as much about these types of genes as their normal function and the mechanism involved in silencing them,” said Dr. Mostafa. “So it was very rewarding that CNOT3 is an important factor in keeping them off.”
The messenger RNA Connection:
Further investigations into the underlying cellular mechanisms revealed a surprising connection between CNOT3 and the messenger RNA of these normally deactivated genes. Messenger RNA (mRNA) is a single-stranded molecule that corresponds to the genetic sequence of a gene and is essential for the synthesis of proteins.
Under normal circumstances, the mRNA of these genes hardly expresses. After CNOT3 was removed, the researchers found that the mRNA was much more stable. In fact, protein has been made from the stabilized mRNA, which has an adverse effect on normal tissue function. This suggests that at least one way to keep these genes switched off is to destabilize their mRNA, which is controlled by CNOT3.
“This study is a step towards understanding the molecular mechanisms that control normal beta cell function,” said Dr. Mostafa. “Ultimately, it could open up new ways to prevent and treat diabetes.”
###
Reference: “Loss of β-cell identity and the diabetic phenotype in mice due to disruption of CNOT3-dependent mRNA deadenylation” by Dina Mostafa, Akiko Yanagiya, Eleni Georgiadou, Yibo Wu, Theodoros Stylianides, Guy A. Rutter, Toru Suzuki and Tadashi Yamamoto August 28, 2020, Communication biology.
DOI: 10.1038 / s42003-020-01201-y
In addition to Dr. Mostafa also Dr. Akiko Yanagiya and Professor Tadashi Yamamoto from the OIST Cell Signal Unit, Dr. Eleni Georgiadou and Professor Guy A. Rutter von Imperial College London, Dr. Yibo Wu and Dr. Toru Suzuki from the Riken Center of Integrative Medical Sciences and Dr. Theodoros Stylianides from Loughborough University.