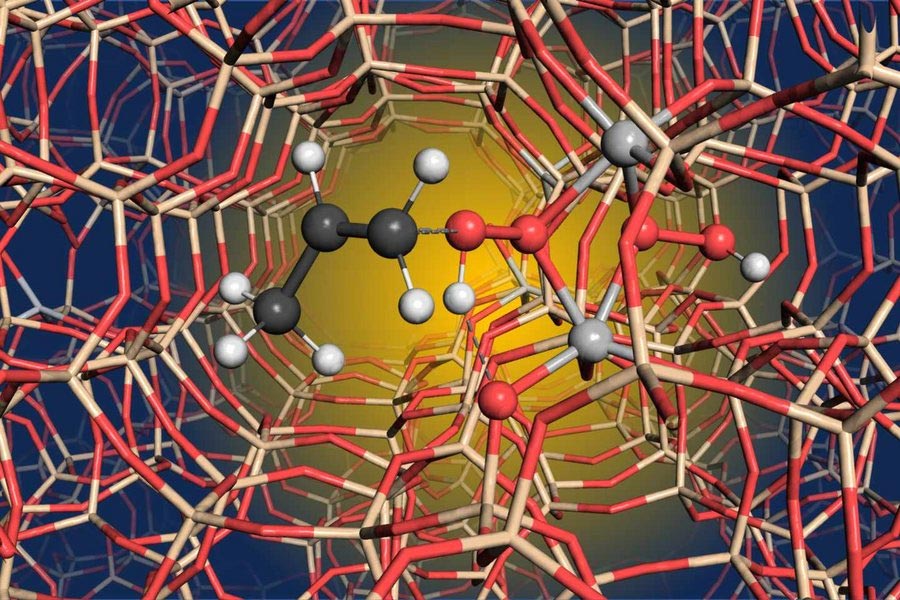
3D model of the active center of the titanium silicalite -1 catalyst with the titanium pair (light gray). Photo credit: ETH Zurich
The activity of the industrial catalyst TS-1 is based on titanium pairs / Important discovery for catalyst development.
‘Titanium Silicalite-1’ (TS-1) is not a new catalyst: almost 40 years have passed since its development and the discovery of its ability to convert propylene to propylene oxide, an important basic chemical in the chemical industry. By combining different methods, a team of scientists from ETH Zurich, the University of Cologne, the Fritz Haber Institute and BASF has now revealed the surprising mechanism of action of this catalyst. The working group of Professor Dr. Albrecht Berkessel involved in the chemistry department. These findings will help catalyst research to take an important step forward.
Propylene oxide is used in industry to manufacture products such as polyurethanes, anti-freeze additives and hydraulic fluids. In the chemical industry, more than 11 million tons of propylene oxide are produced annually, of which 1 million tons are already produced by oxidizing propylene with hydrogen peroxide. This chemical reaction is catalyzed by TS-1, a microporous, crystalline material made of silicon and oxygen that contains small amounts of titanium. The catalyst has been used successfully for 40 years and experts assumed that the active center in TS-1 contains individual isolated titanium atoms, which ensure the special reactivity of the catalyst.
A team of researchers from ETH Zurich, the University of Cologne, the Fritz Haber Institute and BASF challenged this assumption. “In recent years, doubts have arisen as to whether the assumption about the mechanism of action is correct, as it is based mainly on analogies with comparable catalysts and less on experimental evidence. However, trying to optimize a catalyst based on a wrong assumption is very difficult and can lead you in the completely wrong direction. It was therefore important to examine this assumption more closely, ”explains BASF scientist Dr. Henrique Teles, one of the co-authors of the scientific publication, the starting point for the collaboration.
In a study published in natureUsing solid-state NMR studies and computer models, the team was able to show that two neighboring titanium atoms are required to explain the respective catalytic activity. This in turn led the research team to the conclusion that the titanium atoms are not isolated, but that the catalytically active center consists of a titanium pair. “None of the methods used in the study are fundamentally new, but none of the research groups involved in the study could have carried out the investigation on their own,” emphasizes Prof. Christophe Copéret from ETH Zurich, the corresponding author of the publication. “Only the combination of different subject areas and different techniques made it possible to examine the active center of the catalyst more closely.”
“We worked for many years to elucidate the reaction mechanism of a homogeneous titanium catalyst and found that, contrary to the assumptions in the literature, the hydrogen peroxide is activated by a titanium pair. It was really a special moment when we saw in the current study that the results of homogeneous catalysis also apply to heterogeneous catalysis, ”said co-author Prof. Albrecht Berkessel from the University of Cologne. And Dr. Thomas Lunkenbein, co-author of the Fritz Haber Institute in Berlin, adds: “We are very pleased that we were able to make a contribution to this study. We were able to support the conclusions with our analysis. Knowledge of a diatomic active center is fundamental and opens up new possibilities in catalyst research. “
The team is convinced that the results of this study not only contribute to improving existing catalysts, but also to developing new homogeneous and heterogeneous catalysts.
Reference: “Efficient epoxidation over binuclear sites in titanium silicalite-1” by Christopher P. Gordon, Hauke Engler, Amadeus Samuel Tragl, Milivoj Plodinec, Thomas Lunkenbein, Albrecht Berkessel, Joaquim Henrique Teles, Andrei-Nicolae Parvulescu and Christophe Copéret, 28 October 2020, nature.
DOI: 10.1038 / s41586-020-2826-3