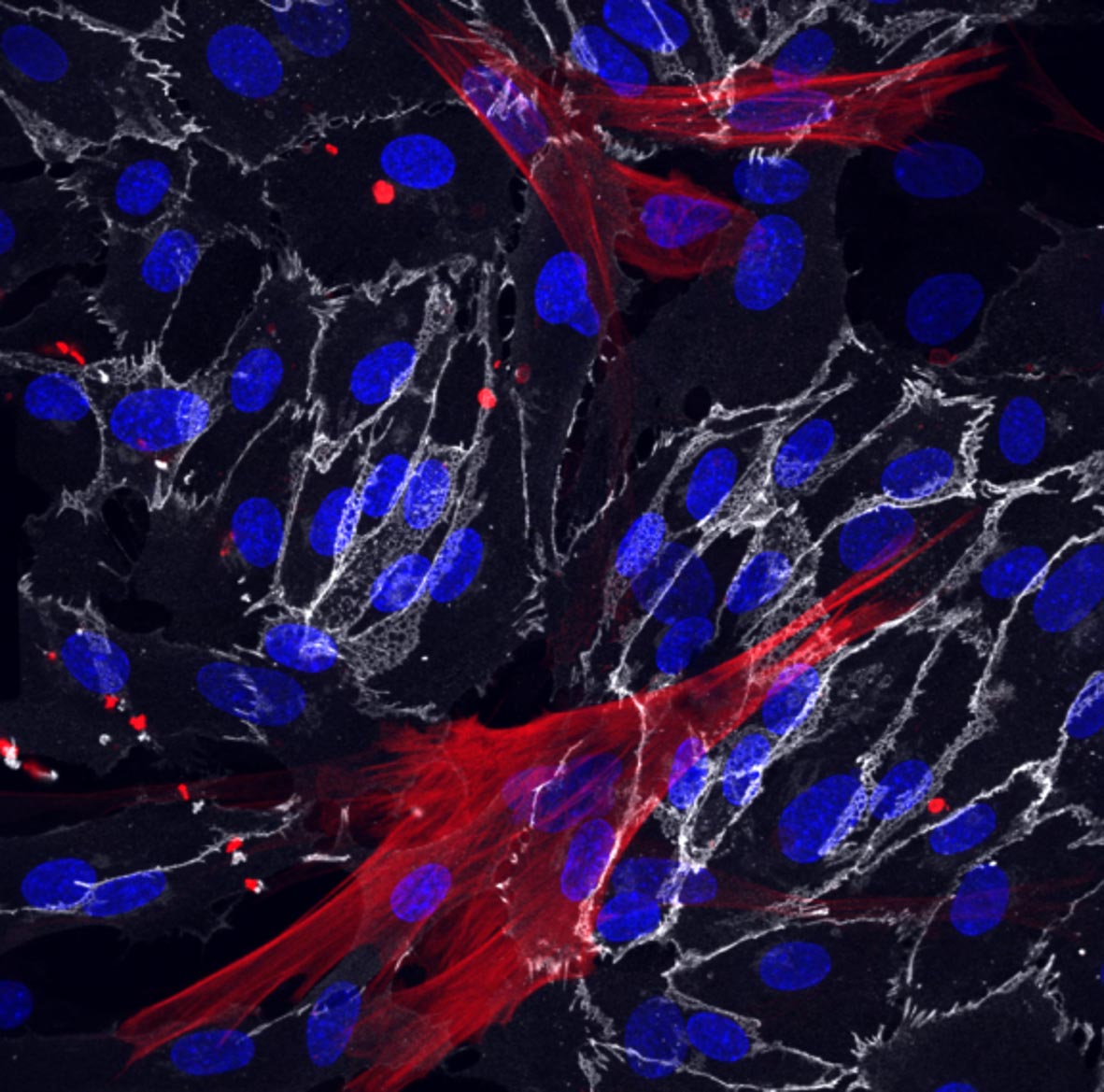
Skin fibroblasts have been successfully reprogrammed into the smooth muscle cells (red) and endothelial cells (white) that surround the blood vessels. The cell nuclei are shown in blue. Photo credits: Bersini, Schulte et al. CC at 4.0
The Salk study is the first to show how cells from the human circulatory system change with age and age-related diseases.
Salk scientists have used skin cells called fibroblasts from patients young and old to successfully create blood vessel cells that retain their molecular age markers. The team’s approach, described in the journal eLife On September 8, 2020, evidence was found of why blood vessels leak and harden with age, and researchers can identify new molecular targets that may slow aging in vascular cells.
“The vasculature is extremely important to aging, but its effects have been underestimated because it has been difficult to study how these cells age,” said Martin Hetzer, senior writer for the newspaper and vice president and chief science officer of Salk.
Research into aging vessels has been hampered by the fact that the collection of blood vessel cells from patients is invasive. However, when blood vessel cells are created from special stem cells called induced pluripotent stem cells, age-related molecular changes are wiped away. Most of the knowledge about how blood vessel cells age comes from observing how the blood vessels themselves change over time: veins and arteries become less elastic, thicken and stiffen. These changes can lead to an increase in blood pressure and an increased risk of heart disease with age.

From left: Martin Hetzer and Simone Bersini. Photo credit: Salk Institute
In 2015, Hetzer was part of the team led by Salk President Rusty Gage to show that fibroblasts can be reprogrammed directly into neurons, skipping the induced pluripotent stem cell stage that erases the cells’ aging signatures. The resulting brain cells retained their age markers and had the researchers study how neurons change with age.
In the new work, Hetzer and colleagues used the same direct conversion approach to create two types of vascular cells: vascular endothelial cells, which make up the inner lining of blood vessels, and the smooth muscle cells that surround these endothelial cells.
“We are among the first to use this technique to investigate the aging of the vascular system,” says Roberta Schulte, Hetzer laboratory coordinator and co-first author of the paper. “The idea to develop these two cell types from fibroblasts was out there, but we adapted the techniques to our needs.”
The researchers used skin cells obtained from three young donors ages 19-30, three senior donors ages 62-87, and 8 patients with Hutchinson-Gilford Progeria Syndrome (HGPS), a common accelerated premature disorder Aging, collected were studying aging.
The resulting induced vascular endothelial cells (iVECs) and induced smooth muscle cells (iSMCs) showed clear age signatures. 21 genes have been expressed at various levels in the iSMCs of old and young people, including genes related to the calcification of blood vessels. 9 genes were expressed differently in the iVECs depending on age, including genes caused by inflammation. In patients with HGPS, some genes reflected the same expression patterns normally seen in the elderly, while other patterns were unique. In particular, levels of BMP-4 protein, which are known to play a role in blood vessel calcification, were slightly higher in aged cells compared to younger cells, but significantly higher in smooth muscle cells from progeria patients. This suggests that the protein is especially important in accelerated aging.
The results not only suggested how and why blood vessels change with age, but also confirmed that the direct conversion method of creating vascular endothelial and smooth muscle cells from patient fibroblasts allowed the cells to maintain age-related changes.
“One of the biggest theoretical implications of this study is that we now know that we can look at an individual patient lengthways as they age or during treatment, to see how their vasculature changes and how we can potentially target them,” says he Simone Bersini, postdoc at Salk and co-first author of the work.
To test the usefulness of the new observations, the researchers tested whether blocking BMP4 – which was found in higher levels in smooth muscle cells developed by people with HGPS – might help treat aging blood vessels. In smooth muscle cells from donors with vascular disease, antibodies that blocked BMP4 decreased vascular leakage – one of the changes that occur with aging vessels.
The results suggest new therapeutic targets for the treatment of both progeria and the normal age-related changes that can occur in the human vasculature. They also illustrate that the direct conversion of fibroblasts into other mature cell types – previously successful in neurons and now in vascular cells – is likely useful in studying a wide range of aging processes in the body.
“By repeating what has been done with neurons, we’ve shown that this direct reprogramming is a powerful tool that can likely be applied to many cell types to study aging mechanisms in all sorts of other human tissues,” says Hetzer, owner of the Jesse and Chairman of the Caryl Philips Foundation.
The team plans future studies to examine the precise molecular mechanisms by which some of the genes that change with age control changes in the vasculature.
Reference: “The direct reprogramming of human smooth muscles and vascular endothelial cells reveals defects related to aging and Hutchinson-Gilford-Progeria syndrome” by Simone Bersini, Roberta Schulte, Hannah Tsai, Ling Huang and Martin W. Hetzer, 8. September 2020, eLife.
DOI: 10.7554 / eLife.54383
Other researchers on the study were Ling Huang and Hannah Tsai from Salk. The work was supported by grants from the National Institutes of Health, the NOMIS Foundation, and an AHA Allen Brain Health and Cognitive Impairment Initiative jointly awarded by the American Heart Association and the Paul G. Allen Frontiers Group. Simone Bersini was supported by the Paul F. Glenn Center for Biology of Aging at the Salk Institute.