Ben Kothe / Information; Getty Photos (3)
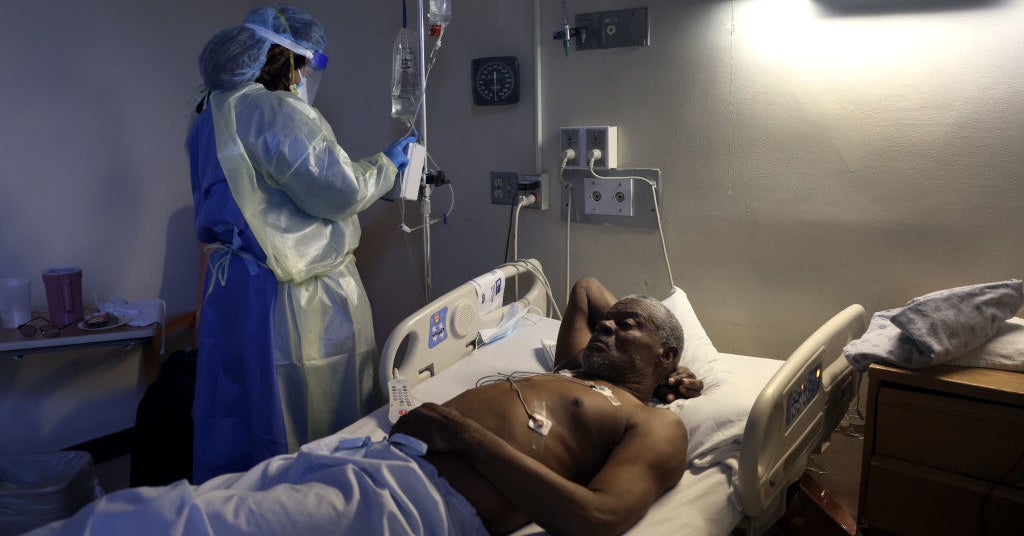
At the same time as vaccines roll out, the pandemic’s holy grail — a drug to efficiently deal with COVID-19 — continues to elude medication.
On Monday, a World Well being Group panel known as for scientists to stop researching hydroxychloroquine, the poster little one for medication that didn’t work in opposition to the coronavirus. In the meantime, greater than 40,000 individuals are nonetheless hospitalized nationwide with COVID-19, and solely a handful of mediocre therapies will help deal with them. And with new variants threatening to thwart vaccines, discovering medication to struggle SARS-CoV-2 is all of the extra pressing.
“The underside line of what we have to do trying ahead, and the clear want on this, is the event of potent antivirals straight appearing on SARS-CoV-2,” Anthony Fauci, chief of the Nationwide Institute of Allergy and Infectious Ailments, stated at a White Home briefing final week. Antivirals would revolutionize the struggle in opposition to SARS-CoV-2, since they block viruses from replicating and may cease individuals from getting very sick or dying.
However efforts to develop such medication have languished due to an absence of funding and coordination: Whereas Operation Warp Velocity devoted almost $18.75 billion to develop vaccines, it solely put aside $6.34 billion for medication. As a substitute, scientists tried to repurpose older medication, together with antivirals for different ailments, to see in the event that they labored in opposition to COVID-19.
“Everybody was searching for a fast repair,” Fauci instructed Information. The FDA has thus far solely approved one drug to deal with COVID-19, remdesivir, initially developed in opposition to Ebola. However it’s removed from an ideal drug: Outcomes on the way it impacts the size of hospital stays have been combined, and it has not been proven to cut back deaths.
“There’s nothing mistaken with searching for fast fixes, however you will additionally need to make the long-term funding,” Fauci stated, noting the seek for efficient new medication would take anyplace from months to a 12 months. The purpose could be to develop medication which can be explicitly designed and focused to SARS-CoV-2 just like the “spectacularly profitable” ones made in opposition to HIV and hepatitis C, which made the lethal ailments treatable, he stated.
However that’s not occurring but. After evaluating a whole lot of older medication, the Nationwide Institutes of Well being has no new antivirals for COVID-19 in its public-private partnership of medical trials, known as Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). There are no newly designed antivirals listed among the many 160 NIH-supported medical trials registered by the Nationwide Library of Medication. Operation Warp Velocity’s “medical countermeasures” effort additionally comprises no new antivirals.
The one new therapies on the ACTIV checklist are monoclonal antibodies — like those former president Donald Trump took — that are troublesome to offer sufferers as a result of they require hourlong transfusions close to the beginning of an sickness. (“HHS is working to extend consciousness of the therapeutics and supply [monoclonal antibodies] to extra varieties of websites,” in line with an company assertion. Company figures counsel monoclonal antibody use for outpatients at excessive threat of growing extreme COVID-19 has risen to roughly 34%, up from 20% in December.)
Scientists have tried to repurpose older medication to assist deal with COVID-19 — with out a lot success.
Probably the most profitable repurposed drug within the pandemic has been dexamethasone, a steroid invented in 1957 — so way back that Fauci stated he prescribed it in graduate college. As a substitute of attacking the coronavirus, dexamethasone suppresses the immune system, which may flip the physique in opposition to itself and assault important organs within the later phases of COVID-19. This low cost and protected drug lowered deaths amongst COVID-19 sufferers on ventilators by a few third, a compelling argument to search for one other needle within the haystack of older medication that might work straight in opposition to the virus.
“There’s lots of causes to make use of these FDA-approved medicines, as a result of they’ve already recognized properties in human sufferers,” stated Texas A&M biochemist Wenshe Ray Liu, who’s researching each new and repurposed medication to struggle the coronavirus. And testing them is comparatively straightforward: Corporations simply need to dose contaminated cells in check tubes with their library of current medication and pull out those that appear to dam SARS-CoV-2. As a result of they’ve already been vetted, the winners can go straight into medical trials with out intensive research to indicate they’re protected.
Medical trials are costly, Liu added, and pharmaceutical corporations desire to check medication they’ve already spent cash to develop. For each of these causes, drug corporations have largely pursued small medical trials with too few sufferers to indicate that their very own medication labored, hoping to hit a house run. These sufferers have little incentive to enroll to check the experimental medication on the early phases of the illness after they aren’t terribly sick.
Molnupiravir, an antiviral designed to struggle the flu, is an instance of the challenges dealing with medical trials to check promising medication. Security trials this summer time seemed promising for the drug, and the drug firm Merck began a bigger medical trial of 1,300 sufferers in October to see if it is going to decrease virus ranges. It’s anticipated to finish in December 2021, taking three to 4 occasions longer than vaccine trials that enrolled tens of hundreds of individuals. On prime of different challenges, the examine measures diminished viral masses reasonably than improved sufferers’ signs, which won’t persuade the FDA of its advantages.
These sorts of hurdles are one motive that repurposed medication haven’t proven a lot profit in sufferers. And whereas NIH-funded trials are learning greater than a dozen outdated medication for ailments corresponding to arthritis, most cancers, malaria, hepatitis, or gout to see if they will struggle SARS-CoV-2, the one antiviral in addition to remdesivir in ACTIV’s medical trials is a Japanese pancreatitis drug developed within the Nineteen Eighties. Outcomes from that trial, which began final August, are projected for the top of this 12 months.
Outcomes from smaller trials for molnupiravir ought to come sooner, stated College of North Carolina virologist Victor Garcia-Martinez, who led a February examine displaying the drug was very efficient in mice geared up with human lung tissue. Ridgeback Biotherapeutics, which is growing the drug with Merck, offered some preliminary outcomes from that trial in 202 hospitalized adults at a scientific convention on Saturday morning. These confirmed a faster lower in virus ranges on nasal swabs of sufferers who took the drug, in comparison with those that did not. The agency hopes to current full outcomes displaying whether or not the drug utterly eradicated the virus quicker at an upcoming medical assembly.
If it will definitely proves efficient, Garcia-Martinez stated, “This can be straightforward to fabricate and distribute. Significantly if there may be an outbreak, say, in a nursing dwelling, you will get it straight away to individuals.”
Scott Olson / Getty Photos
The coronavirus has traits that make it laborious to search out an efficient drug to struggle it — however there may be motive to be hopeful.
Many medication commonly hobble viruses in check tubes, even different coronaviruses, stated drug researcher Martin Michaelis of the College of Kent in the UK. Scientists have been initially optimistic that these medication would equally work in opposition to SARS-CoV-2, however this virus is totally different sufficient that almost all of these medication will not be efficient.
In a current examine, Michaelis and colleagues laid out the variations between SARS-CoV-2 and its closest human-infecting relative, the SARS virus, which killed 774 individuals after the 2002 outbreak. Whereas they’re about 80% related genetically, the 2 viruses differ within the organic equipment they use to copy inside cells, Michaelis and his colleagues discovered. That’s essential as a result of interfering with this viral replication is a predominant activity of antiviral medication. If viruses can’t replicate, they will’t unfold.
Though the spikes on the surface of the coronavirus at the moment are mutating wildly to supply extra contagious variants, the virus has much less freedom to vary its reproductive course of, Michaelis stated. That’s as a result of the identical equipment can be important in different primary viral capabilities, making it a extra dependable goal for medication taking goal on the virus.
“You may’t say these conserved areas won’t ever mutate,” he stated, “however it’s a decrease likelihood.”
Examples of recent antivirals designed particularly in opposition to SARS-CoV-2 at the moment are turning up in animal research. A Feb. 18 report by Chinese language researchers within the journal Science discovered that two medication efficiently diminished viral masses within the lungs of mice.
And there are different indicators we might discover useful COVID-19 medication. A 12 months into the pandemic, scientists know way more about how SARS-CoV-2 works and could also be higher geared up to search out current medication that might assault it, Texas A&M’s Liu stated. His group lately discovered a hypertension drug that in a pc simulation matches like a key in a lock to the coronavirus. The drug might have been missed as a result of it’s a generic and doesn’t have a company sponsor prepared to put money into a medical trial.
“We’ll attempt to begin our personal medical trial if we are able to’t discover a company sponsor,” Liu stated.
Andrew Caballero-reynolds / Getty Photos
Extra money is required to push as laborious on therapies as we did on vaccines.
On March 11, 2020, the day that the pandemic was first declared by the WHO, Fauci testified to Congress that there have been two avenues for medication to deal with the coronavirus: vaccines or medication.
From a scientific perspective, there have been lots of causes to have anticipated an efficient antiviral drug to show up before a vaccine, Fauci instructed Information. “You typically know comparatively shortly whether or not a [drug] remedy works or not since you’re giving a remedy to any person that is already sick,” he stated.
A vaccine, in the meantime, requires giving actual pictures and placebos to tens of hundreds of individuals after which ready till pure infections trigger sufficient infections to indicate it’s efficient.
“We have been lucky in that we had a few vaccine candidates that have been red-hot and actually turned out to be good,” Fauci stated. “And sadly for the nation, however thankfully for the vaccine trials, we continued to have a excessive degree of an infection, which allowed us to get a solution fairly shortly.”
Vaccines set off a well-understood pure immune response, making their design and security testing extra simple in comparison with new antivirals, Michaelis added. However the US vaccine trials additionally had considerably extra funding than ones for therapies.
“There may be not that a lot cash in antiviral medication for acute ailments which can be solely used for every week or so,” Michaelis stated. The shortage of a transparent path means new antivirals designed explicitly in opposition to the coronavirus will doubtless require a giant public funding, Fauci stated. The NIH solely lately began an initiative to analysis new antivirals, which might be “unlikely to supply therapeutics in 2021,” NIH chief Francis Collins instructed the New York Occasions.
In some methods, the dearth of COVID-19 antivirals underscores simply how fortunate humanity is to have efficient vaccines, Michaelis stated.
“{People} tried many, many compounds in opposition to SARS-CoV-2, COVID-19, however they only haven’t had the massive, large, profitable candidate thus far,” he stated. Forging forward to create new medication, he added, “turns into more durable and tougher work.”
UPDATE
UPDATE
Dikkat: Sitemiz herkese açık bir platform olduğundan, çox fazla kişi paylaşım yapmaktadır. Sitenizden izinsiz paylaşım yapılması durumunda iletişim bölümünden bildirmeniz yeterlidir.
#Vaccines #COVID19 #Dont #Good #Therapies
Supply: buzzfeednews