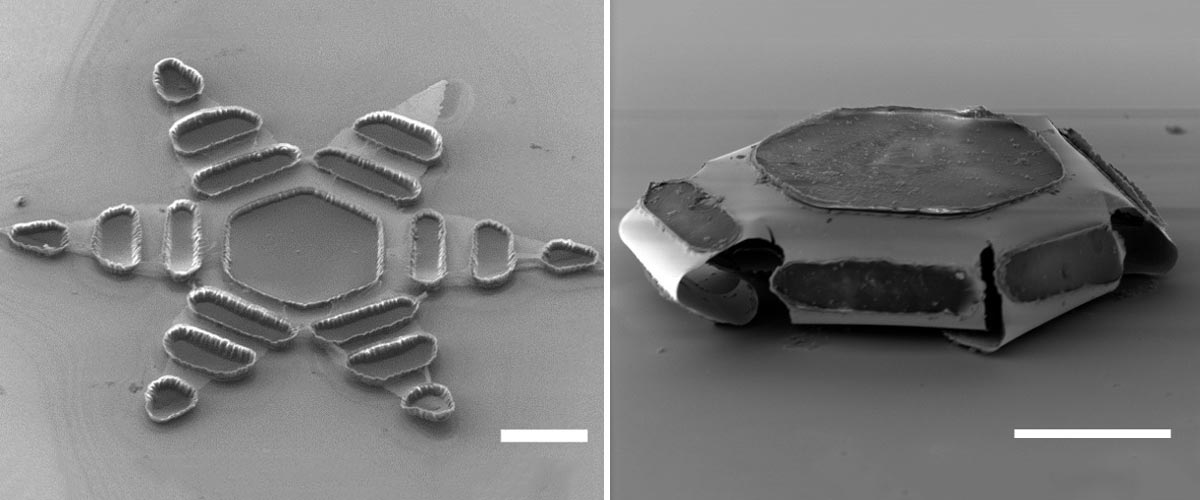
When an open zipper is left exposed to internal body temperatures, it closes on the intestinal wall. In the middle of the gripper there is a space for a small dose of a drug. Photo credit: Johns Hopkins University
“Theragripper” are inspired by a parasitic worm that clings to its host’s intestines.
Inspired by a parasitic worm that burrows its sharp teeth into its host’s intestines, Johns Hopkins researchers have developed tiny, star-shaped micro-devices that attach to the lining of the intestine and deliver drugs into the body.
David Gracias, Ph.D., Professor in the Whiting School of Engineering at Johns Hopkins University, and Dr. med. Florin M. Selaru, Johns Hopkins gastroenterologist, director of the Johns Hopkins Inflammatory Bowel Disease Center, led a team of researchers and biomedical engineers designed and tested shape-changing micro-devices that mimic the way the parasitic hookworm attaches itself to an organism’s gut .
Made of metal and thin, shape-changing film and coated with a heat-sensitive paraffin wax. Theragrippers, each roughly the size of a dust spot, can potentially carry any medicine and gradually release it into the body.
The team recently published results from an animal study as a cover article in the journal Advances in science.
Gradual or prolonged release of a drug has been a long-sought goal in medicine. Selaru explains that one problem with sustained-release drugs is that they often pass all the way through the gastrointestinal tract before they have given off their medication.

A Theragripper is about the size of a dust spot. This swab contains dozens of the tiny devices. Photo credit: Johns Hopkins University
“Normal narrowing and relaxation of the muscles of the gastrointestinal tract makes it impossible for extended-release drugs to stay in the bowel long enough for the patient to receive the full dose,” says Selaru, who has worked with Gracias for more than 10 years . “We have worked to solve this problem by developing these small drug carriers that can autonomously attach themselves to the intestinal mucosa and hold the drug load in the GI tract for a desired time.”
Thousands of zippers can be used in the GI tract. When the paraffin wax coating on the grippers reaches body temperature, the devices close autonomously and clamp themselves to the wall of the colon. The closing action causes the tiny, six-pronged devices to dig into the lining and stick to the colon, where they are retained and gradually release their drug payloads into the body. Eventually the Theragripper lose their grip on the tissue and are removed from the intestine via normal gastrointestinal muscle function.
Gracias notes advances in biomedical engineering in recent years.
“We have seen the advent of dynamic, microfabricated smart devices that can be controlled by electrical or chemical signals,” he says. “But these grippers are so small that batteries, antennas and other components cannot fit on them.”
Theragripper, says Gracias, doesn’t rely on electricity, wireless signals, or external controls. “Instead, they work like small compression springs with a temperature-controlled coating on the devices that autonomously releases the stored energy at body temperature.”
The Johns Hopkins researchers manufactured the components with around 6,000 thermal grippers per 3-inch silicon wafer. In their animal experiments, they loaded a pain reliever drug onto the claws. The researchers’ studies found that the animals given Theragripper had higher concentrations of the pain reliever in their bloodstream than the control group. The drug remained in the test subjects’ systems for almost 12 hours versus two hours in the control group.
Reference: “Gastrointestinal Shape-Modifying Micro Devices Prolong Drug Release In Vivo” by Arijit Ghosh, Ling Li, Liyi Xu, Ranjeet P. Dash, Neha Gupta, Jenny Lam, Qianru Jin, Venkata Akshintala, Gayatri Pahapale, Wangqu Liu, Anjishnu Sarkar, Rana Rais, David H. Gracias and Florin M. Selaru, October 28, 2020, Advances in science.
DOI: 10.1126 / sciadv.abb4133
The authors of the journal article are Gracias and Selaru Arijit Ghosh, Liyi Xu, Neha Gupta, Qianru Lin, Gayatri Pahapale, Wangqu Lu and Anjishnu Sarkar from the Department of Chemical and Biomolecular Engineering at Johns Hopkins University. Ling Li and Venkata Akshintala from the Department of Gastroenterology and Hepatology, Johns Hopkins University School of Medicine; Ranjeet Dash, Jenny Lam, and Rana Rais of Johns Hopkins Drug Discovery and the Department of Neurology at Johns Hopkins University School of Medicine.
The work was funded by the National Institute for Biomedical Imaging and Bioengineering at the National Institutes of Health and the National Science Foundation. Johns Hopkins University has filed patents relating to this technology on behalf of Gracias and Selaru, in accordance with the university’s conflict of interest guidelines.
The technology can be licensed through Johns Hopkins Technology Ventures.